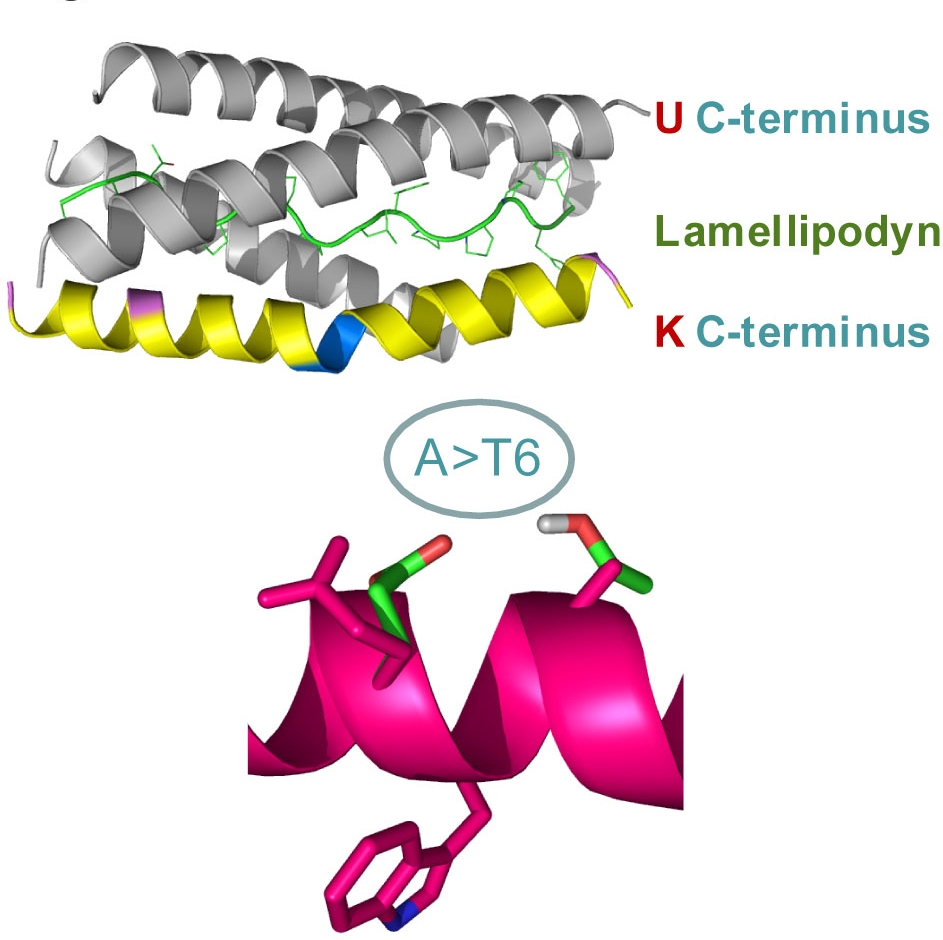
Figure 2.
Structural effects of alanine-to-tryptophan substitution in position 539 of BChE. Shown is molecular modeling of the helical C-terminal peptides of the “usual” (wild-type) BChE (gray) and the BChE-K variant (yellow), as these interact with the proline-rich Lamellipodin peptide with which BChE is associated in the serum. Note that the A-to-T mutability, characteristic of the K variant, and which is schematically drawn below, induces a kink. This impairs protein-protein interactions of the BChE-K C-terminal peptide, possibly by changing the positioning of the adjacent tryptophan – as we could experimentally validate by nuclear magnetic resonance measurements.26